What is Nystagmus?
Nystagmus is a visual condition marked by involuntary, rhythmic eye movements, often described as “jumping” or “dancing” motions. These movements can affect both eyes or just one, though it’s more frequent they affect both.
The involuntary eye movements may appear as either rapid or slow motions, with constant speed or with acceleration/deceleration phases, exhibiting various patterns such as for example horizontal motions. As a result, individuals with nystagmus may struggle to maintain steady focus on objects, leading to reduced visual acuity. Additionally, if acquired in adulthood, these movements can also impact balance and coordination.
Classification
There are many types of nystagmus but we will discuss deeply only nystagmus that can be of interest for Blue Cone Monochromacy. We will not write here about all other types of nystagmus, for example physiological nystagmus-– normal nystagmus variant of oculomotor function – or pathologic nystagmus results from diseases affecting the cortex, anterior visual tracts, central nervous system, brainstem, cerebellum, and peripheral vestibular apparatus.
For Blue Cone Monochromacy we are interested in benign nystagmus usually seen in infancy, particularly on the Infantile Nystagmus Syndrome (INS; formerly known as “congenital” nystagmus (CN)).
Others types of benign nystagmus usually seen in infancy are the nystagmus of the fusion maldevelopment nystagmus syndrome (FMNS, formerly known as latent/ manifest latent nystagmus (LMLN)), and the pendular nystagmus of the Spasmus Nutans Syndrome (SNS).
Blue Cone Monochromacy, Achromatopsia and Infantile Nystagmus Sydrome
In Blue Cone Monochromacy, the first symptom observed in all 2-3 months old newborn babies is involuntary horizontal oscillations of both eyes toward and away from an attempted point of fixation. In many babies this oscillation decreases and ceases at the end of the first year, and in this case it is not currently known how to classify this type of nystagmus, for example whether to call it Spasmus Nutans or not. There are no clinical studies done up to now on this form of nystagmus.
A fraction of patients reports that nystagmus persists into adulthood and in this case the oscillations can be classified – following Dr. Dell’Osso [1]- as Infantile Nystagmus Syndrome (INS), formerly known as Congenital Nystagmus. Preliminary results of the BCM Families Foundation Patient Registry data report that in the same family 45% of affected individuals show nystagmus and 55% don’t show nystagmus in adulthood.
If persistent into adulthood, in individuals with Blue Cone Monochromacy nystagmus can lead to challenges with reading and with other tasks. Individuals with Blue Cone Monochromacy report that nystagmus becomes worse in moments of stress, illness, or fatigue.
In Achromatopsia patients report that the involuntary horizontal oscillations of both eyes toward and away from an attempted point of fixation start at 2-3 months in newborn babies and then decreases at the end of the first year. However, nystagmus persists in all adults with Achromatopsia although it appears only in moment of stress, illness, or fatigue [2].
Here is a video of a 4-months baby with Blue Cone Monochromacy showing Nystagmus. However, in this case, the nystagmus lasted beyond the first year of age and didn’t disappears clinically in childhood, therefore it can be classified as Infantile Nystagmus Syndrome.
Here is a video of an adult affected by BCM with Nystagmus – he is patient III-2 of Pedigree 1 below:
What is Infantile Nystagmus Syndrome (INS) and other types of benign form of nystagmus in infancy and childhood
Infantile Nystagmus Syndrome (INS), sometimes also referred to with obsolete terms as “Early-Onset Nystagmus” or “Congenital Nystagmus (CN)”, appears at birth or emerges within the initial months of life during the various sensitive periods defining the development of visual fixation, and it persists throughout life. As it is important in Blue Cone Monochromacy, we want to stress here that INS is a lifelong condition.
Typically, infants begin exhibiting symptoms between six weeks and three months old. Infantile Nystagmus Syndrome can may be triggered by any various neurological and medical conditions which are detectable through tests done by a neurologist.
INS is usually horizontal, with a small torsional component and may (rarely) have a vertical component. The intensity of INS increases on lateral gaze and becomes right beating in right gaze and left beating in left gaze. INS remains horizontal in up gaze (in contrast to acquired and/or vestibular nystagmus, which changes direction in vertical gaze); INS increases intensity with fixation attempt or stress and decreases with sleep or inattention; INS has variable intensity in different positions of gaze (usually about a null position); INS changes direction in different positions of gaze (about a neutral position); INS has decreased intensity (damps) with convergence; INS exhibits anomalous head posturing; INS could be associated with strabismus; and INS could be associated with an increased incidence of significant refractive errors.
Similarly to what happens for the Blue Cone Monochromacy, INS often occurs in association with congenital or early onset (first 6 months of life) acquired defects in the visual sensory system (e.g., systemic and ocular albinism, achromatopsia, aniridia, congenital retinal dystrophies and degenerations, visual cortex anomalies, and congenital cataracts, glaucoma, and corneal diseases) and in this case was previously named “sensory” nystagmus.
Fusion maldevelopment nystagmus syndrome (FMNS)—formerly known as latent/manifest latent nystagmus (LMLN) in the case of monocular deprivation. There is always associated strabismus and ocular motor recordings show four types of slow phases with jerk in direction of fixing eye. The oscillations appear conjugate, horizontal, uniplanar and there are usually no associated sensory system deficits (eg, no albinism, no achromatopsia, no blue cone monochromacy). Patients with FMNS always have strabismus and, to eliminate diplopia, vision from the tropic eye is suppressed (“occluded”) in the cortex. FMNS occurs in patients with strabismus who, although viewing with both eyes open, are fixing monocularly. Strabismus is a necessary (but not sufficient) condition for FMNS. Various theories have been advanced to explain FMNS. We will not discuss further FMNS because it is not related with Blue Cone Monochromacy or Achromatopsia.
Spasmus Nutans Syndrome. The Spasmus Nutans Syndrome (SNS) includes ocular oscillations, head nodding, and anomalous head positions that begins in infancy (usually between 4 and 18 months of age) and disappears clinically in childhood (usually before 3 years or age). The nystagmus is generally bilateral (but can differ in each eye and may even be strictly monocular), and it oscillates in horizontal, torsional, or vertical directions. The average duration of SNS is 12 to 24 months; rarely, it lasts a number of years. In the case of Blue Cone Monochromacy we should carry out further studies to understand whether the nystagmus that appears around 6-8 weeks and disappears forever at one year of age can be classified as SNS or not. A clinical study would be needed.
Acquired Nystagmus
Acquired nystagmus emerges later, starting as early as six months of age but potentially occurring at any time afterward, with a higher incidence observed in adults. It can have many etiologies and it also may be linked to medical conditions warranting further evaluation by medical professionals. We want to stress here that acquired causes of nystagmus require exploration, if the eye movements develop later in life, by medical professionals.
Associated Conditions
A list of conditions possibly triggering INS includes:
- Inherited conditions (such as Blue Cone Monochromacy, Achromatopsia and Leber Congenital Amaurosis)
- Albinism
- Genetic deficits
A list of conditions possibly triggering Acquired Nystagmus includes:
- Congenital cataracts
- Inner ear disorders
- Neurological disorders
- Systemic disorders
- Visual problems
- Inflammatory diseases
- Medications and drugs use
- Trauma
- Stroke
- Brain tumor
Recognizing the underlying diseases associated with nystagmus is crucial for effective management and treatment. As such, we strongly recommend that a comprehensive assessment by healthcare experts, such as ophthalmologists and neurologists, in order to determine associated diseases and develop an appropriate management plan tailored to the individual’s needs.
Perceptions
Children and adults affected by Infantile Nystagmus Syndrome, as a large percentage of individuals with Blue Cone Monochromacy, typically perceive the world similarly to other people without nystagmus, albeit with some degree of blurriness. Conversely, individuals with nystagmus that develops in adulthood often describe a sensation of movement or “shaking” in their visual field, known as oscillopsia.
To Learn More
Causes of Infantile Nystagmus Syndrome (INS)
INS is caused by a failure of the ocular motor system, the part of the brain that controls eye movement, to keep the eyes stable during the pursuit of a slow target. See ref [1] Chapter 1 and Ref. [4] for an introduction to the ocular motor system.
There are four basic types of eye movements – ref [1] Chapter 1 -:
- saccades, that are ballistic, conjugate eye movements that in a fast way change the point of foveal fixation. These are the fastest eye movement, 250-800°/sec with 200milli sec of latency. For example, you use saccades to point a target, to make a rapid re-fixation of a stimulus. It allows you to identify a target in the visual field with voluntary ocular movement and bring it into the fovea, carrying out foveation;
- smooth pursuit movements, are much slower tracking movements of the eyes designed to keep a moving stimulus on the fovea. For example, you use smooth pursuit movements when you follow the horizontal or vertical movement of a slow target; 0-30° per sec with a latency of 125 milli sec;
- vergence movements, that is disjunctive movements of the eyes (convergence or divergence) that align the fovea of each eye with targets located at different distances from the observer. You use vergence when you fix on an approaching or receding target; and
- vestibulo-ocular movements, that stabilize the eyes relative to the external world, thus compensating for head or body movements. If you fix a target and turn the head you are using these movements. Optokinetic movement is similar, and tracks images of the world during sustained world or background motion.
The accurancy of movements 1) – 4) are not affected by INS and the efficacy and quality of the movement is almost the same of individuals with and without INS. During periods of foveation, individuals with INS can maintain fixation as precisely as people without nystagmus [3]. However, we note here that vergence movements are able to stop nystagmus.
Nystagmus is related to movements 2). Let’s consider the smooth pursuit system inside the eye motion system.
The pursuit system is a delicate system with a damped oscillation. Once the target has been inserted on the fovea – foveation – the eye fixes it – fixation – and if the target moves making movements with various low speeds and accelerations, the eye is able to follow the target keeping it on the fovea to see it in detail and chasing it, adapting to its speed. The developing ocular motor system (OMS) requires good visual input to calibrate properly, and INS arises from a failure to calibrate a subsystem that is already borders on instability (i.e., oscillatory behavior) in normal humans. This failure to calibrate can be triggered or be associated with inherited or acquired medical conditions.
Let’s see what is a damped oscillation.
If you have a mass attached to a spring, with respect to the equilibrium position at x=0, moving the mass by an amplitude A, in the absence of friction, it will begin an oscillation around the point x=0 with a period T.

We note that we can visualize this motion in the next figure, with the case b=0 (absence of viscous friction) as a harmonic motion, in which during time t the mass m continuously oscillates between +A and -A. The extreme points +A and -A correspond to a velocity of the mass equals to 0 and to a reversal of the motion. The point x=0 corresponds to the point of maximum speed. In our eye system, the point x=0 is the target, and the oscillation is the nystagmus. Nystagmus cannot be considered a compensatory symptom of a sensory deficit, because it produces a vision handicap, given that the the eye oscillates between 0 and maximum velocity while fixating a stationary target.

Red line: over damped system
Orange line: critical – this is the fastest route without oscillation
Blue Line: under damped system
In individuals without nystagmus the smooth pursuit system is calibrated to have a damping force such as viscous friction, capable of damping the oscillation and tracking the target as it moves.
There are several models of the smooth pursuit system that have been developed, see for example Ref.[4] . These models try to obtain the velocity of the eye as a function of the velocity of the target, and they try to reproduce the high performance of this system to pursuits a slow moving target and its short latency time between the stimulus movement and the eye response, that is the eye velocity.
Can Blue Cone Monochromacy or other retinal diseases be considered a cause of nystagmus?
No. Some genetic diseases of the retina, such as Blue Cone Monochromacy and Achromatopsia, lead to low vision from birth. Individuals affected by these pathologies may present involuntary eye movements during the first year of life which in some subjects persist into adulthood and can be classified as Infantile Nystagmus Syndrome (INS). According to Dell’Osso it is not possible to detect a cause and effect condition between visual impairment and INS, because for example, for the same retinal disease there are individuals who show nystagmus and individuals who do not have it.
For example, we report the pedigree of two families affected by Blue Cone Monochromatism, a disease transmitted on the X chromosome, for which an average of approximately 5 individuals per family are affected according to data from the International Patient Register www.BCMRegistry.org. From the pedigrees we note that a large fraction of individuals, approximately 45%, show INS.



As explained by Dell’Osso, a sensory deficit is a condition that can trigger nystagmus but is not the only cause, otherwise these individuals would all be affected (i.e., the condition is not sufficient). Also, since others individuals have INS without a sensory deficit, it is not a necessary condition; being neither a necessary nor a sufficient condition, it cannot be the cause.
At the begin of this webpage we reported the video of nystagmus in patient III-2 of Pedigree 1.
Gene Therapy and Nystagmus
Genetic diseases of the retina such as Leber’s Congenital Amaurosis, Blue Cone Monochromacy and Achromatopsia are currently interested by the development of gene therapies through sub-retinal or intra-vitreal injection of AAV viral vectors capable of reinserting into cells of the retina the missing proteins. The relevant question is whether an improvement in nystagmus can be seen after gene therapy and how this is achieved.
Initial evidence shows an improvement in individuals treated with Luxturna for RPE65 LCA. We report here a picture from Ref. [5].
Scientists looked at nystagmus using digital video data of eye movements for qualitative and quantitative clinical analysis of the subject’s oscillation and strabismus. They also measured amplitudes/frequency of nystagmus in movies of pupillary light reflex.

Picture from Ref. [5]
After injection of AAV2.hRPE65v2 gene therapy, the patients had a decrease in both monocular and binocular amplitude and frequency of nystagmus in primary position, with eccentric gaze, and with monocular cover. See Table 1 of Ref.[6] “Characteristics of the Patients and Test Results before and after Injection”.
In conclusion is seems possible to have a reduction in the severity of nystagmus after gene therapy, that is, is it possible that after gene therapy a re-calibration of the OMS might occur.
Regarding Blue Cone Monochromacy, it will be important to discuss nystagmus and inclusion/exclusion criteria for a gene therapy clinical trial. If we expect a reduction in nystagmus after gene therapy injection, then nystagmus cannot be an exclusion criteria for participating in a gene therapy trial because individuals with nystagmus should have an added benefit from the therapy. A similar investigation needs to be done for strabismus.
As nystagmus and strabismus can be treated with surgery, another questions is whether having done a nystagmus or strabismus surgery is an exclusion criteria for participating in a gene therapy trial.
Muscles and Cranial Nerves involved in Nystagmus
The coordination of eye movements, including fixation, involves a complex interplay of neural circuits within the brainstem and higher brain centers. These circuits integrate sensory input, visual information, and motor commands to ensure precise control of eye movements and maintain steady gaze on a specific object or point of interest. There are six pairs of eye muscles and three pairs of cranial nerves that control eye muscles involved on eyes movements.
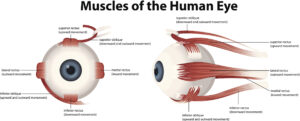
Eye Muscles
There are six muscles that enable the eye movement, the superior, inferior, medial and lateral rectus and the superior and inferior oblique:
Cranial Nerves
There are 12 sets of Cranial Nerves (CN). Among them, there are three pairs of cranial nerves that control muscles of eye movement (each eye is controlled by one nerve of each pair). Damage to these nerves can interfere with eye muscles, and are associated with nystagmus. This is not the case of the nystagmus in Blue Cone Monochromacy and Achromatopsia, where usually there aren’t damages in Cranial Nerves. The rest of the nerves in the body emerge from the spinal cord but all the cranial nerves come from the brain.
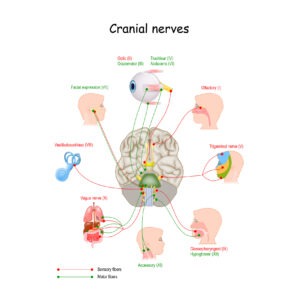
- The oculomotor nerve (cranial nerve three, CNIII) controls several muscles that move eyes: the superior rectus muscle, the medial rectus muscle, the inferior rectus muscle, and the inferior oblique muscle. These muscles move your eyes straight up and down and toward your nose. CNIII allows not only movement of the eye muscles, but also constriction of the pupil, focusing the eyes and the position of the upper eyelid.
- The trochlear nerve (cranial nerve four, CNIV) controls the superior oblique muscle that moves your eye in a direction that is down and away from your nose.
- The abducens nerve (cranial nerve six, CNVI) controls the lateral rectus muscle, which moves your eye outward and away from your nose.
- The vestibulocochlear nerve (cranial nerve eight) mediates your sense of sound and balance. It does not control eye movement, but a deficit in this nerve can impair balance to a degree that causes nystagmus.
- The trigeminal nerve, also called the cranial nerve V. The trigeminal nerve has three branches that perform distinct functions, one of these is the ophthalmic branch that sends nerve impulses from the upper part of your face and scalp to your brain. The ophthalmic nerve relates to your eyes, upper eyelids and forehead. It is important for the proprioception, explaining why dressing contact lenses can reduce nystagmus.
Eyes and Head movements, and Null Point
In the case of bilateral visual reduction, and when the visual deficit arises in the first months of life, as in Blue Cone Monochromacy, the nystagmus is conjugated – that is, there is the same movement in both eyes – and predominantly pendular – horizontal with constant speed without acceleration or deceleration – in type, is reduced by convergence and is often accompanied by head swaying, side-to-side movements.
The intensity of the eye movements in nystagmus can vary depending on the direction of the individual’s gaze. Therefore, as reduced eye shaking implicates better vision, a way in which some of the individual affected by nystagmus manage to optimize their vision is by adjusting their head to access their null point. This term refers to a specific head position where the involuntary eye movements are minimized, allowing for reduced oscillations of their eyes and improved visual acuity. While not all individuals with nystagmus have a null point, if they do, it’s important not to encourage them to turn their head in the opposite direction, as this could worsen their vision. Head movements, known as “null point” or “nulling” maneuvers, are voluntary or involuntary actions that individuals with nystagmus may adopt to minimize the intensity or frequency of nystagmus oscillations and improve visual acuity. Some individuals may have a null point in primary position, that is without turning their head, in a straight-ahead position. When it is determined that the null point is not in the primary position, prismatic lenses may be used or eye muscle surgery may be considered, the latter even in cases of primary-position nulls.
Then, the null position is that range of gaze angles where the nystagmus waveform has minimal amplitude and has the highest foveation quality. However, turning the head to reach the null point is not the only head movement that people with Blue Cone Monochromacy and nystagmus can have. There are several types of head movements commonly associated with nystagmus:
- Head Turn: Some individuals with nystagmus may instinctively turn their head to one side or the other to find a position where nystagmus movements are minimized. This head turn can help align the eyes in a way that reduces the amplitude of nystagmus oscillations and improves visual clarity.
- Head Tilt: Another common head movement observed in individuals with nystagmus is head tilting, where the individual tilts their head to one side or the other. Similar to head turning, head tilting aims to find a position that minimizes the intensity of nystagmus movements and optimizes visual function.
- Nodding: Some individuals with nystagmus may exhibit nodding movements, where they rhythmically nod their head up and down. Nodding movements can alter the dynamics of nystagmus and may be a compensatory strategy to stabilize gaze and improve visual acuity; it may also reflect head oscillations driven by the neck muscles in response to copies of the eye-muscle signals
These head movements are often adopted spontaneously by individuals with spasmus nutans as they seek to mitigate the effects of nystagmus on their vision.
It’s important to note that not all individuals with nystagmus exhibit head movements, and the presence and extent of head movements can vary depending on factors such as the severity and type of nystagmus, individual preferences, and adaptation strategies developed over time. Additionally, head movements in response to nystagmus may be more pronounced in certain viewing conditions or activities where visual demands are heightened.
Stress and Fatigue
Nystagmus becomes worse in moments of stress or fatigue as reported by many members of BCM community, and of the Achromatopsia community The exact cause is unknown –that is the relationship between stress, fatigue, and nystagmus is unknown.
We note here that:
Increased Visual Demands: Stressful situations often involve heightened visual demands, such as focusing on detailed tasks or processing complex visual information. Nystagmus may become more noticeable or disruptive in these situations as the visual system is challenged to maintain steady gaze and clear vision under stress. Could following complex reasoning quickly under stress and recalling complex processes with brain memory involve a visualization aspect of the process and with it nystagmus?
Fatigue and Reduced Concentration: Fatigue can impair concentration and cognitive function, leading to decreased ability to suppress or compensate for nystagmus movements. As fatigue sets in, individuals may experience diminished control over eye movements, resulting in increased nystagmus activity.
It is very important to address this aspect to teachers of children with BCM to explain them how to assist their student with BCM to function well in class despite his nystagmus, for example being aware that the student needs to manage his level of stress and fatigue, allowing rest periods, possibly a few minutes after each task that requires greater effort in visual concentration. Please find here more information.
Strabismus and Blue Cone Monochromacy
Some individuals affected by Blue Cone Monochromacy report strabismus, some other report both nystagmus and strabismus.
Treatment of Nystagmus
Consulting with medical professionals specializing in neuro-ophthalmology can offer valuable perspectives and resources for further exploration.
There is a variety of surgical therapies that will damp INS in specific patients without harming (and in many cases, improving) ocular motor and visual function. They include: 1) the Kestenbaum (Anderson-Kestenbaum) procedure; 2) the Anderson plus T&R procedure; 3) the BMR procedure; 4) the T&R procedure; 5) procedures on the vertical rectus and oblique muscles in combination with these INS procedures; and 6) strabismus procedures in combination with these INS procedures. Please refer to expert doctors.
Here we report some ophthalmologists expert on nystagmus surgery and treatment:
https://www.akronchildrens.org/people/Richard-Hertle-MD.html
http://www.omlab.org/Personnel/lfd/lfd.html
Glossary
Oscillopsia
Perception of oscillopsia (world movement), that is is a sensation of the environment moving back and forth. Patients with INS do not normally experience oscillopsia, while patients who acquire nystagmus in adulthood experience oscillopsia.
Foveation
When with a saccadic movement you to identify a target in the visual field with voluntary ocular movement and bring it into the fovea, you are carrying out foveation. Target, head & body, or background motion foveation is maintained by the fixation, through the smooth pursuit, vestibulo-ocular, or optokinetic systems respectively.
Fixation
Fixation refers to the ability of the eyes to maintain steady gaze on a specific object or point of interest. Individuals with normal vision typically have excellent fixation abilities, characterized by the ability to maintain steady gaze on a specific object or point of interest with minimal eye movements. In individuals with normal vision, the visual system can easily maintain steady fixation, allowing for clear and stable visual perception, which is crucial for tasks such as reading, driving, and navigating the environment.
Extrafoveal fixation
Extrafoveal fixation refers to the use of peripheral vision rather than central vision (fovea) to perceive visual information. When central vision is affected by the underlying visual abnormalities, extrafoveal fixation can be particularly beneficial. By relying on peripheral vision, individuals with central vision diseases can bypass some of the challenges associated with central vision deficits and achieve better overall visual function. Extrafoveal fixation allows for a broader field of view and can compensate for reduced visual acuity or difficulties with foveal fixation.
Saccadic movements
Ballistic, conjugate eye movements that change the point of foveal fixation. For example you use saccades to point a target.
External Resources
- American Association for Pediatric Ophthalmology and Strabismus – https://aapos.org/glossary/nystagmus
- American Nystagmus Network – https://nystagmus.org/
Cleveland Clinic – https://my.clevelandclinic.org/health/diseases/22064-nystagmus - Fighting Blindness – https://www.fightingblindness.ie/living-with-sight-loss/eye-conditions/nystagmus/
- Johns Hopkins Medicine – https://www.hopkinsmedicine.org/health/conditions-and-diseases/nystagmus
- Dr. Luis F. Dell’Osso Lab – http://www.omlab.org/
References:
[1] Richard W. Hertle and Louis F. Dell’Osso “Nystagmus in infancy and childhood” Oxford University Press 2013 Chapter 3 and its references.
[2] Associazione Acromati Italiani – patients reported data
[3] 2020 Jun;140(3):221-232. Epub 2019 Nov 27. “Foveation dynamics in congenital nystagmus IV: vergence” Louis F Dell’Osso, Johannes Van Der Steen, Robert M Steinman, Han CollewijnPMID: 31776760 DOI: 10.1007/s10633-019-09738-y
[4] John Enderle, Models of Horizontal Eye Movements, Part I: Early Models of Saccades and Smooth Pursuit January 2010 Synthesis Lectures on Biomedical Engineering 5(1) DOI:10.2200/S00263ED1V01Y201003BME034 Publisher: Morgan & Claypool Publishers.
[5] Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010 Mar;18(3):643-50. doi: 10.1038/mt.2009.277. Epub 2009 Dec 1. PMID: 19953081; PMCID: PMC2839440.
[6] Albert M. Maguire, M.D., Francesca Simonelli, M.D., Eric A. Pierce, M.D., Ph.D., Edward N. Pugh, Jr., Ph.D., Federico Mingozzi, Ph.D., Jeannette Bennicelli, Ph.D., Sandro Banfi, M.D., Kathleen A. Marshall, C.O.T., Francesco Testa, M.D., Enrico M. Surace, D.V.M., Settimio Rossi, M.D., Arkady Lyubarsky, Ph.D., Valder R. Arruda, M.D., Barbara Konkle, M.D., Edwin Stone, M.D., Ph.D., Junwei Sun, M.S., Jonathan Jacobs, Ph.D., Lou Dell’Osso, Ph.D., Richard Hertle, M.D., Jian-xing Ma, M.D., Ph.D., T. Michael Redmond, Ph.D.,Xiaosong Zhu, M.D., Bernd Hauck, Ph.D., Olga Zelenaia, Ph.D., Kenneth S. Shindler, M.D., Ph.D., Maureen G. Maguire, Ph.D., J. Fraser Wright, Ph.D., Nicholas J. Volpe, M.D., Jennifer Wellman McDonnell, M.S., Alberto Auricchio, M.D., Katherine A. High, M.D., and Jean Bennett, M.D., Ph.D. “Safety and Efficacy of Gene Transfer for Leber’s Congenital Amaurosis” 2008 N Engl J Med 2008; Vol 358 No 21, 2240-2248 DOI: 10.1056/NEJMoa0802315


